April 4, 2022
Christopher Morabito, MD, CMO
Our goal: To employ our proprietary product engine to systematically identify and validate cellular drug targets that can modulate gene expression to treat the known root cause of genetically defined diseases.
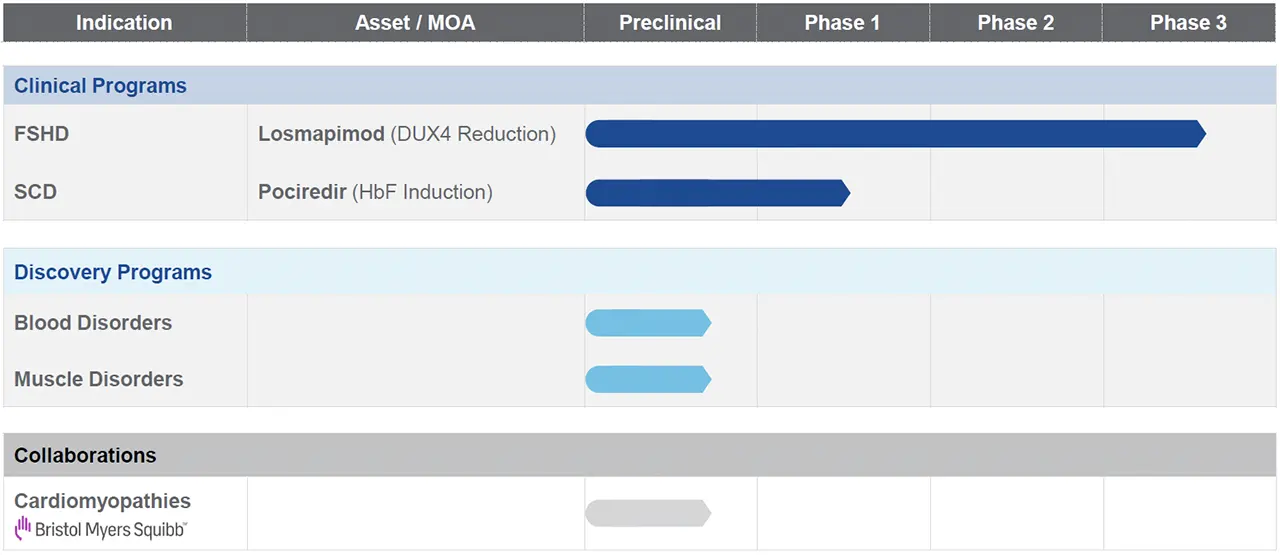
Our approach to drug discovery and development is applicable across a large number of genetically defined diseases. We have developed a rigorous assessment and selection process to determine which of the approximately 7,000 rare, genetically defined diseases we intend to evaluate in drug target identification activities. We are applying our product engine to discover drug targets to modulate gene expression and develop product candidates for the potential treatment of the root cause of these diseases.
By staying true to our passion and always prioritizing patients first, we plan to progress our programs and create significant value for all of our stakeholders.
April 4, 2022
Christopher Morabito, MD, CMO
April 4, 2022
Joost Kools, Nicol Voermans, Karlien Mul, Lucienne Ronco, John Jiang, Jennifer Shoskes, Kelly Marshall, Diego Cadavid, Michelle L. Mellion, Baziel van Engelen
April 4, 2022
Per Widholm, Markus Karlsson, André Ahlgren, Olof Dahlqvist-Leinhard, Rabi Tawil, Kathryn Wagner, Jeffrey Statland, Leo Wang, Perry Shieh, Baziel Van Engelen, Diego Cadavid, Lucienne Ronco, Adefowope Odueyungbo, Jay Han, Maya Hatch, Michelle L. Mellion
April 4, 2022
Reachable Workspace to Evaluate Efficacy of Losmapimodin Subjects with FSHD in Two Phase 2 Studies
Rabi Tawil, MD, Kathryn Wagner, MD, PhD, Jeffrey Statland, MD, Leo Wang, MD, PhD, Angela Genge, MD, Sabrina Sacconi, MD, PhD, Hanns Lochmüller, MD, PhD, David Reyes Leiva, MD, Jordi Diaz-Manera, MD, PhD, Nuria Muelas, MD, PhD, Juan J. Vilchez, MD, PhD, Alan Pestronk, MD, Summer Gibson, MD, Namita A. Goyal, MD, Johanna Hamel, MD, Jay Han, MD, Lawrence Hayward, MD, Nicholas Johnson, MD, Samantha LoRusso, MD, Miriam Freimer, MD, Perry B. Shieh, MD, PhD, Sankarasubramoney H. Subramony, MD, Doris Leung MD, PhD, Baziel van Engelen MD, PhD, Joost Kools, MD, John Jiang, PhD, L. Alejandro Rojas, PhD, Anthony Accorsi, PhD,Christopher Morabito, MD, Jennifer Shoskes, PharmD, Michelle L. Mellion, MD (first author)
April 4, 2022
Rabi Tawil, MD, Kathryn Wagner, MD, PhD, Jeffrey Statland, MD, Leo Wang, MD, PhD, Angela Genge, MD, Sabrina Sacconi, MD, PhD, Hanns Lochmüller, MD, PhD, David Reyes Leiva, MD, Jordi Diaz-Manera, MD, PhD, Jorge Alonso-Perez, MD, Nuria Muelas, MD, PhD, Juan J. Vilchez, MD, PhD, Alan Pestronk, MD, Summer Gibson, MD, Namita A. Goyal, MD, Johanna Hamel, MD, Lawrence Hayward, MD, Nicholas Johnson, MD, Samantha LoRusso, MD, Miriam Freimer, MD, Perry B. Shieh, MD, PhD, Sankarasubramoney H. Subramony, MD, Doris Leung MD, PhD, Baziel van Engelen MD, PhD, Joost Kools, MD, Olof Dahlqvist, Per Widholm, John Jiang, PhD, Adefowope Odueyungbo, PhD, William Tracewell, PhD, Alisa Rahilly, BSc, L. Alejandro Rojas, PhD, Anthony Accorsi, PhD, Steve Mennen, PhD, Lucienne Ronco, PhD, Diego Cadavid, MD, Christopher M. Moxham, PhD, Christopher Morabito, MD, Robert Gould, PhD, Michelle L. Mellion, MD.